Ancora builds complex oligosaccharides using chemical synthesis, in contrast to bioprocess methods that require exhaustive isolation of carbohydrates from fermentation broths. In short, Ancora builds versus isolates. This synthetic approach to antigens affords significant advantages not available with traditional bioprocess production methods, including:
-
Production of high purity, homogeneous carbohydrates with exactly defined structures. Nearly all marketed carbohydrate therapeutics and vaccines are produced by bio-process methods and comprise partially defined mixtures of carbohydrates;
-
Access to otherwise unattainable structures. The desired oligosaccharides often are too difficult to purify using traditional bioprocess methods, leading to high levels of contaminants and high lot-to-lot variability;
-
Access to “core” antigens and defined substructures. Cores have the potential to be excellent vaccine antigens because they are usually conserved across pathogen serotypes and strains; however, these targets are often ignored because until now there has been no reliable means to access them. Defined synthetic substructures proved critical in transforming the bio-process derived anticoagulant enoxaparin (e.g. Lovenox®)into the defined synthetic new chemical entity fondaparinux (e.g. Arixtra®).
-
The ability to optimize carbohydrate structures through iterative rational design and synthesis. Such a “medicinal chemistry” strategy to carbohydrate design cannot be done effectively with bioprocess approaches and is a new concept for vaccines and biotherapeutics fields;
-
The ability to perform systematic structure-activity and epitope mapping studies. Through synthesis and independent testing of discrete defined components of a large polysaccharide, the epitopes critical for biological activity (e.g. protective immune response) may be identified unequivocally. Ancora, in collaboration with Novartis Vaccines (Siena, IT) has demonstrated exactly this for a Candida vaccine candidate (Vaccine 2010 28:2615, Figure A).
-
Downstream development advantages. It is anticipated that defined, homogeneous carbohydrates identified during the preclinical proof-of-concept stage will translate into an improved product CMC dossier supporting regulatory, manufacturing, and safety requirements.
-
Superior conjugation control. Each synthetic oligosaccharide can be produced with a chemically reactive group at a defined site for chemo-specific conjugation to a carrier molecule, biomaterial or surface. In the case of vaccines this leads to more uniform presentation of the antigen to the immune system and better control of antigen loading in the conjugate. Ancora, again in collaboration with Novartis Vaccines, has demonstrated superior conjugation control for a set of Group A Streptococcus vaccine candidates (Vaccine 2010 29:104, Figure B).
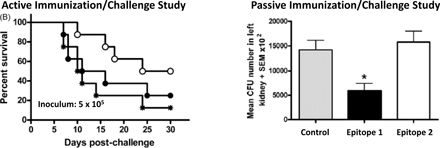
Figure A. Candida Antigen Epitope Mapping. Left panel: Synthetic 15-mer (Epitope 1, ○) protects against death while synthetic branched 17-mer (Epitope 2, ●) does not (Control: MF59 adjuvant alone, ). Right panel: Epitope 1 significantly reduces fungal burden in kidneys while Epitope 2 does not (Vaccine 2010 28:2615).
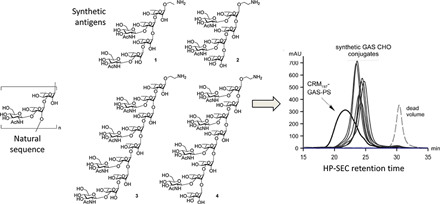
Figure B. (Click to enlarge) Group A Streptococcus Conjugation Profiles. Synthetic antigens (left panel, 1-4) conjugated to CRM197 carrier are more uniform and less polydisperse (right panel, “synthetic GAS CHO conjugates”) compared to the bioprocess-derived polysaccharide conjugate (right panel, “CRM197-GAS-PS”) as shown by high performance size exclusion chromatography (HP-SEC) (Vaccine 2010 29:104).