
Ancora is focused on developing vaccines for the most serious nosocomial (hospital acquired) infections. The nosocomial infection market is significant and growing. In the US alone there are over 250,000 nosocomial bloodstream infections annually associated with 99,000 deaths, making them the 10th leading cause of death in the US. Nosocomial infections are also associated with $45 Billion in direct medical costs (2007, CDC).
Vaccines for nosocomial infections represent a major opportunity, given the absence of currently approved products, the magnitude of the market opportunities, and strong pricing opportunities. For example, the University of Pittsburgh estimated that in hospitals and environments with a high incidence of methicillin-resistant S. aureus (MRSA), a price of $1000 per dose for an effective S. aureus vaccine would still be cost effective.
The opportunity extends far beyond a MRSA vaccine. The top 10 bloodstream nosocomial infections (Table 1) across 49 centers in the US and 24,179 patient admissions provide a glimpse of the breadth of nosocomial vaccine opportunities for Ancora (Wisplinghof et al. 2004):
| Rank | Pathogen | Incidence | % of Bloodstream Infections |
|---|---|---|---|
| 1 | Coagulase-Negative Staphylococcus | 16/10,000 admissions | 31% |
| 2 | Staphylococcus aureus | 10/10,000 | 20% |
| 3 | Enterococcus | 5/10,000 | 12% |
| 4 | Candida species | 4.6/10,000 | 8% |
| 5 | E. coli (pathogenic) | 2.8/10,000 | 6% |
| 6 | Klebsiella | 2.4/10,000 | 5% |
| 7 | Pseudomonas aeruginosa | 2/10,000 | 4% |
| 7 | Enterobacter | 2/10,000 | 4% |
| 9 | Serratia | 1.7/10,000 | 4% |
| 10 | Acinetobacter baumanii | 0.6/10,000 | 1% |
Table 1. Top 10 nosocomial bloodstream infections in the US.
These infections are associated with severe morbidity and high mortality rates, and ever more antibiotic resistant strains continue to emerge.
Ancora envisions targeting specific patient populations for nosocomial vaccines:
Ancora has established a preclinical pipeline of vaccines based on unmet medical need, market potential (Table 2) and technical feasibility.
| Program | Indications | Market Potential |
|---|---|---|
| Staphylococcus | Elective Surgeries (NOS) (cardiovascular, orthopedic) | > $1 Bn |
| Pseudomonas | Ventilator-associated pneumonia (NOS); cystic fibrosis (NOS, COM) | > $1 Bn |
| Enterococcus | Elective Surgeries (NOS) (cardiovascular, orthopedic) | > $0.5 Bn |
| Candida | Invasive candidiasis (NOS); Vaginal candidiasis (COM) | > $0.5 Bn |
| Moraxella | Otitis media (pediatric, COM); COPD (adult, COM) | > $1 Bn |
Table 2. Market potential for Ancora’s nosocomial (NOS) and community-associated (COM) vaccine programs.
The pipeline (see Figure) is heavily focused on nosocomial infections, but also incorporates additional high value opportunities in other areas including community acquired infections and potential bioterrorism threats. In order of priority, the pipeline currently consists of:
Biological function has been demonstrated in three of the current portfolio programs, either by demonstrating immunoprotection in a relevant in vivo disease model and/or by demonstration that antisera raised to the carbohydrate antigens are able to kill the pathogen in vitro. In vivo immunoprotection has also been demonstrated against additional pathogens, including Group A Streptococcus and Plasmodium (the parasite that causes malaria, with the former being conducted in collaboration with Novartis Vaccines and the latter as part of a National Institutes of Health funded program).
The multitude of successful preclinical proofs-of-concept across a wide range of infectious diseases is a strong indication that Ancora’s approach to vaccine antigen production is broadly applicable and is not limited to a select few opportunities. This affords Ancora the luxury of being able to choose and prioritize its vaccine programs based on an overall assessment of each opportunity.
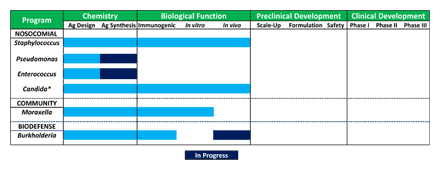
Figure A. (Click to enlarge) Ancora vaccine pipeline.
© 2024 www.ancorapharma.com. All rights reserved.